Clinical Pathology of Sea Turtles
Some in-house testing can easily be performed on fresh blood samples. PCV, TS, and plasma color are very helpful to assess in-house since they provide rapid, valuable information to the veterinarian. Experienced personnel can review blood smears to get an immediate, subjective evaluation of blood cell concentrations and morphology. If samples are sent out to a diagnostic laboratory, it is recommended to use a lab that is experienced with reptiles and to use the same lab routinely (Wolf et al., 2008). It is also recommended to prepare and send at least 2 fresh blood smears upon submission of a blood sample for CBC. It can be helpful to save 2 blood smears on file, which can be stained with Diff-Quik™ or if no stain is available, fixed in 100% methanol for 5 minutes to be saved in slide boxes. A recommendation for blood smear preparation that causes the least traumatic damage to large reptilian blood cells can be found here (PDF) .
Handheld analyzers can be very useful for quick assessment of critical analytes (temperature-corrected blood gases), but it does not provide any reliable hematology results in reptiles (e.g., hematocrit, hemoglobin). If it is impossible to obtain the cloacal temperature of the patient, the ambient temperature can be used for temperature-correction. Most analyzers allow entering the temperature directly, but if necessary, the blood gas results need to be corrected using established formulas (Innis et al., 2007). Please note that the hematocrit and hemoglobin obtained by handheld analyzers are unreliable in reptiles, and only a spun PCV by centrifugation will provide an accurate measurement of the red blood cell mass.
HEMATOLOGY
Since circulating blood cells are all nucleated in reptiles, automated WBC (white blood cell) count methods are not available and manual WBC counts need to be performed (e.g., Natt-Herricks-TIC®, Phloxine B method). The main challenge in reptile hematology is the differentiation between lymphocytes and thrombocytes upon blood film evaluation (Fig. 4) and when performing WBC counts by hemocytometer. Please note: morphologic and quantitative evaluation needs to be performed in areas of even cell distribution on the blood film.
BLOOD CELL MORPHOLOGY
We refer to Stacy NI, 2011 as guideline for cell morphology and cell functions in reptiles in general, the images shown here demonstrate species-specific differences for sea turtles.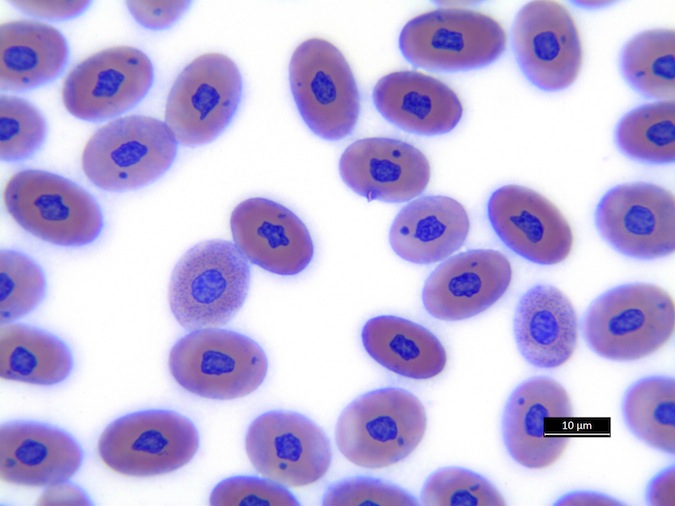
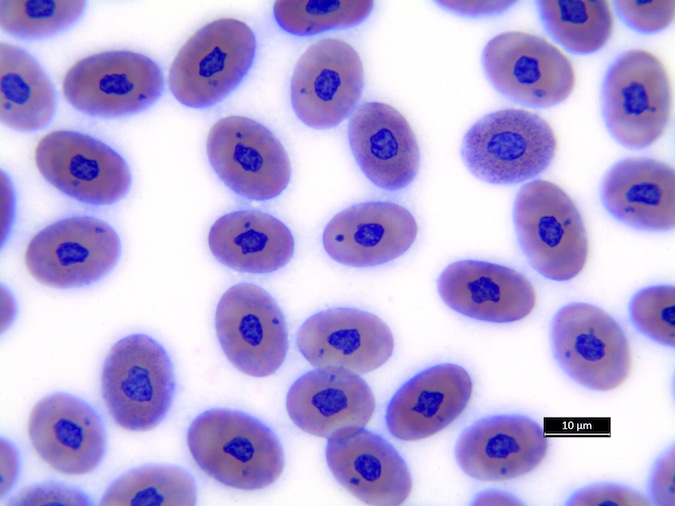
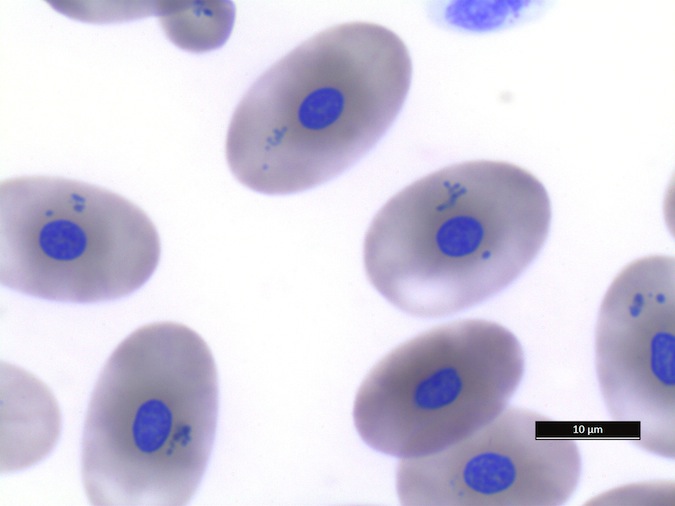
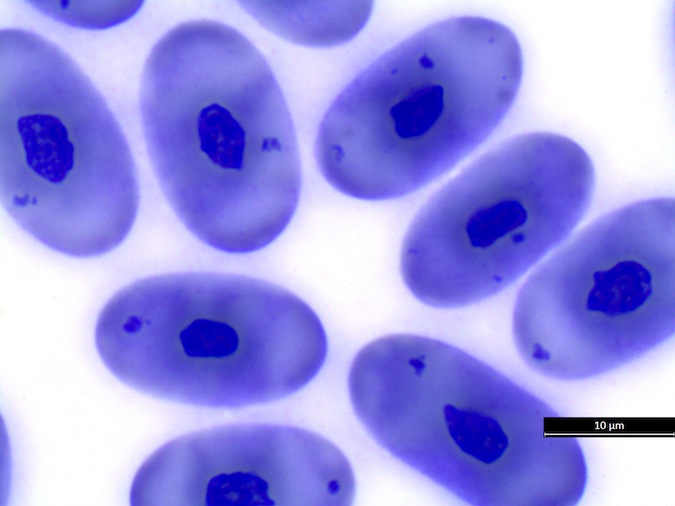
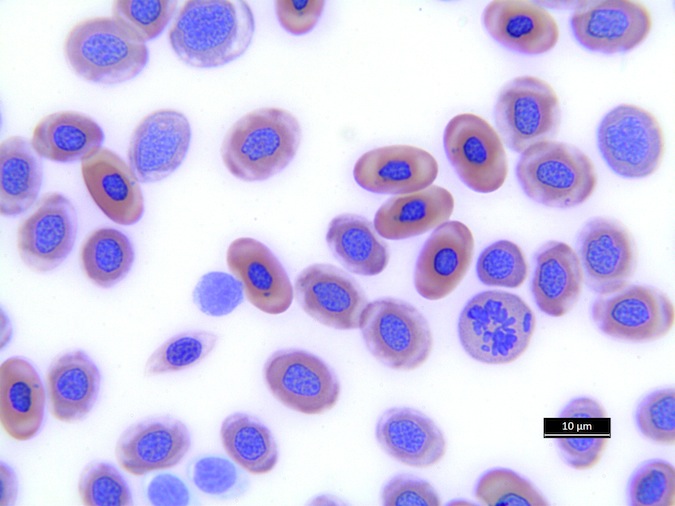
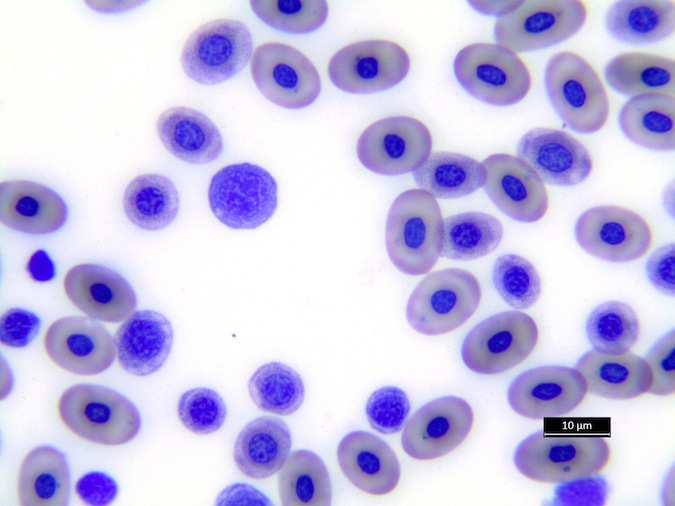
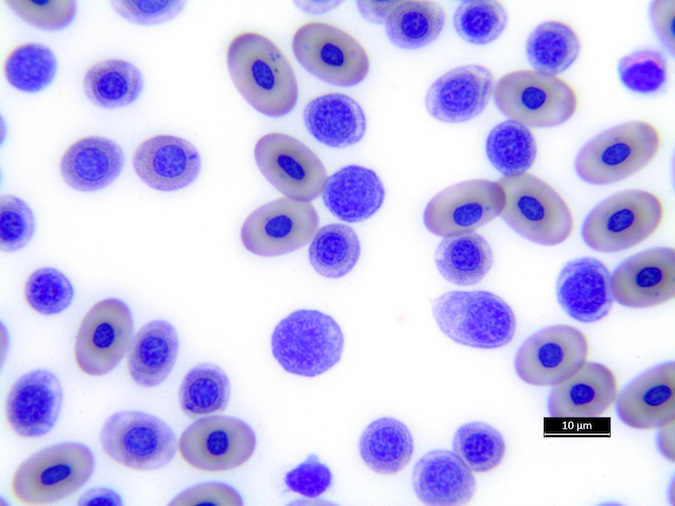
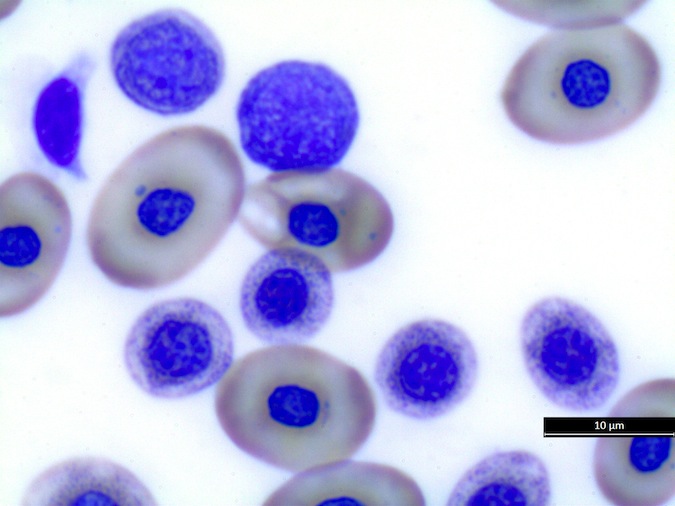
Heterophils are similar in morphology to other reptilian species (Fig. 5) and can also become toxic and/or left-shifted (Fig. 6). Heterophil toxicity is non-specific and can be seen with infections (esp. bacterial), tissue necrosis, metabolic derangements, or with administration of some drugs. In active inflammatory disease, immature (=left-shifted) heterophils can be seen in the peripheral circulation. Left-shifted heterophils can be identified by larger (and thus immature) nuclei that can be elongated or pleomorphic. In some patients, left-shifting and toxicity may be observed concurrently (Fig. 6). The disappearance of toxicity and/or left-shifting are considered good prognostic indicators.
Figure 5. Normal heterophil morphology in sea turtles. Drying artifact visible in heterophils of Eretmochelys imbricata, Caretta caretta, and Lepidochelys olivacea. Wright-Giemsa stain. 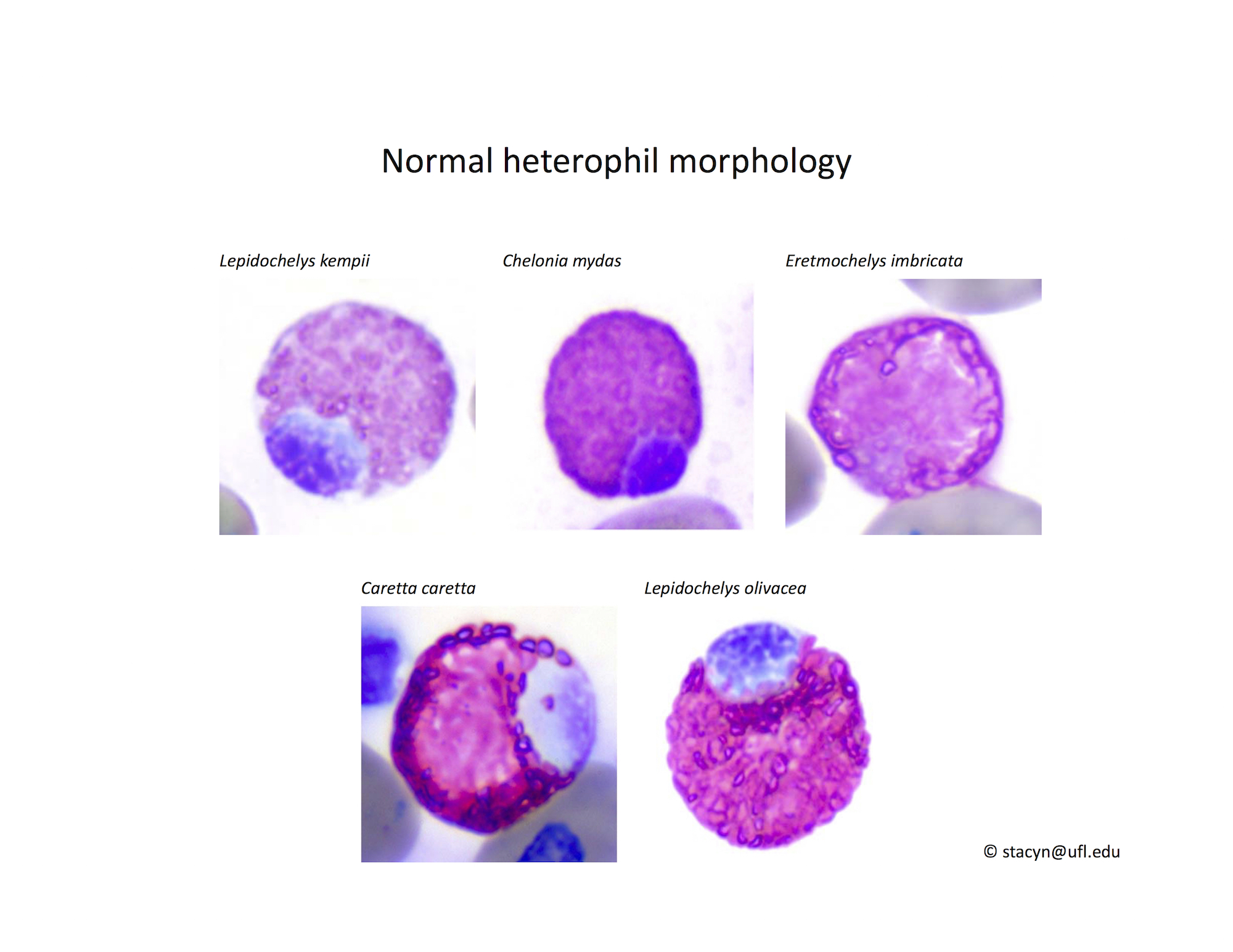
Figure 6. Abnormal heterophil morphology in sea turtles. Wright-Giemsa stain.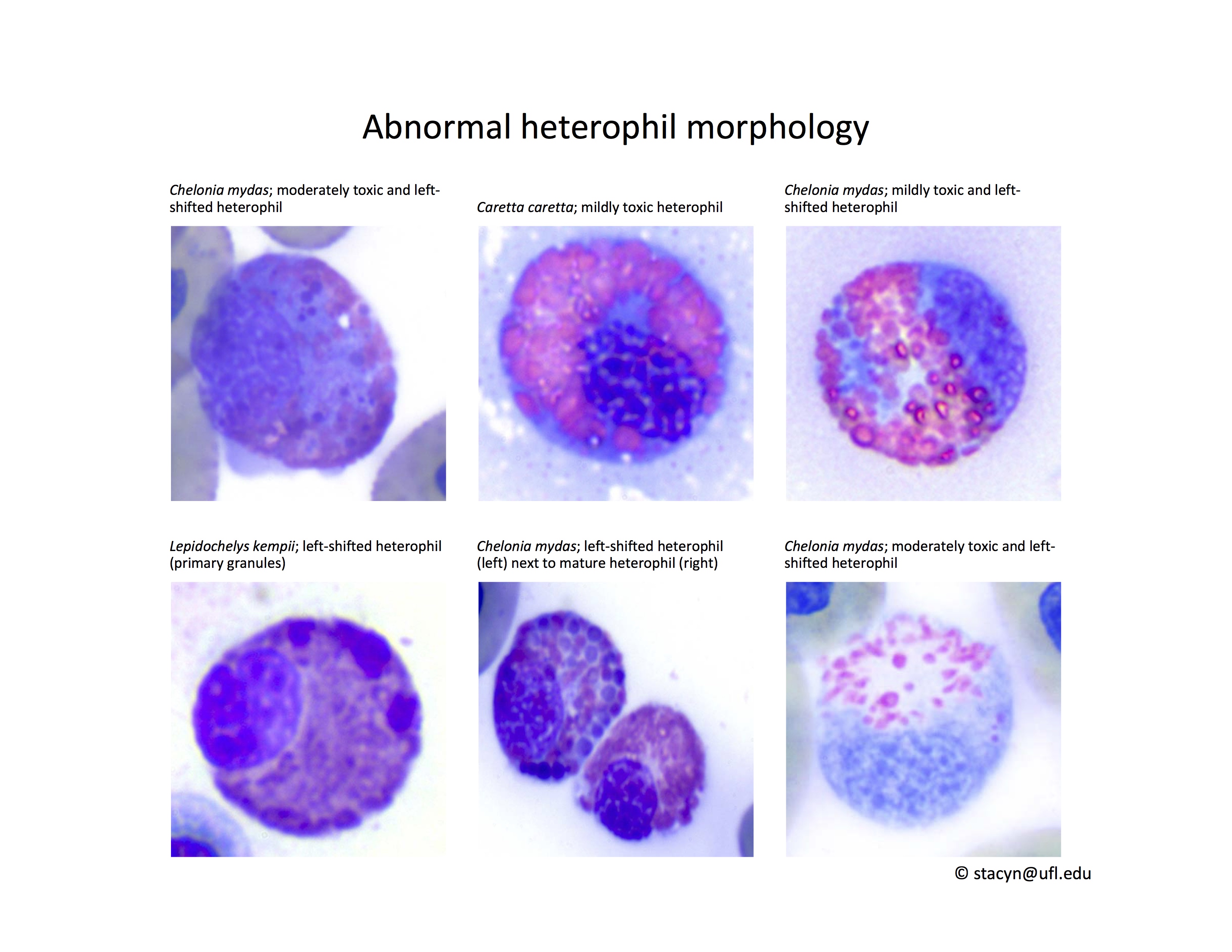
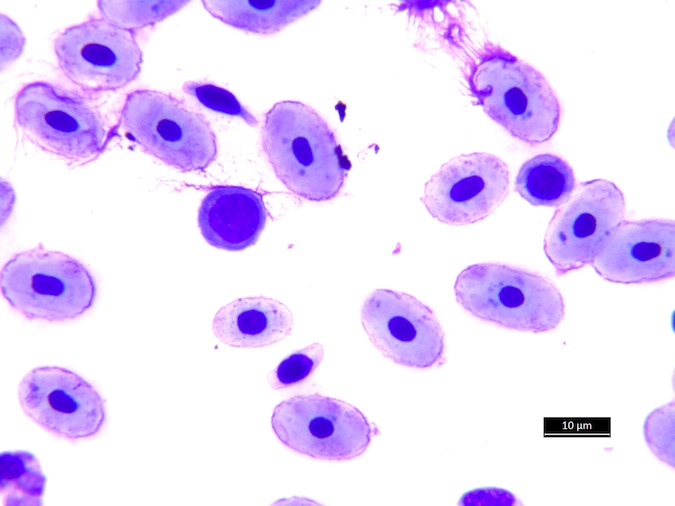
Eosinophils have a unique morphology in sea turtle species, with a variable number of spherical granules that can be focally aggregated within the cell (Fig. 7).
Figure 7. Normal eosinophil morphology in sea turtles. Wright-Giemsa stain.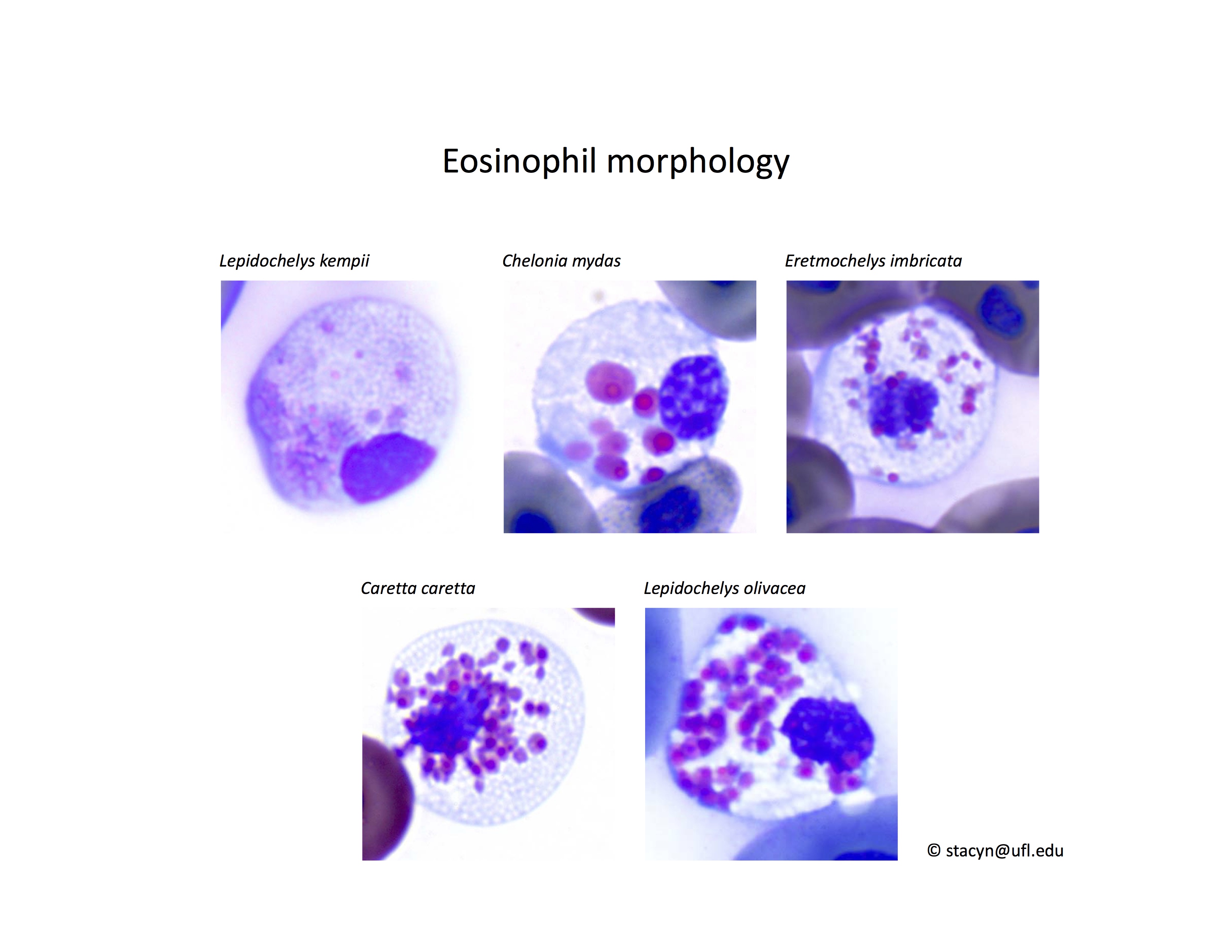
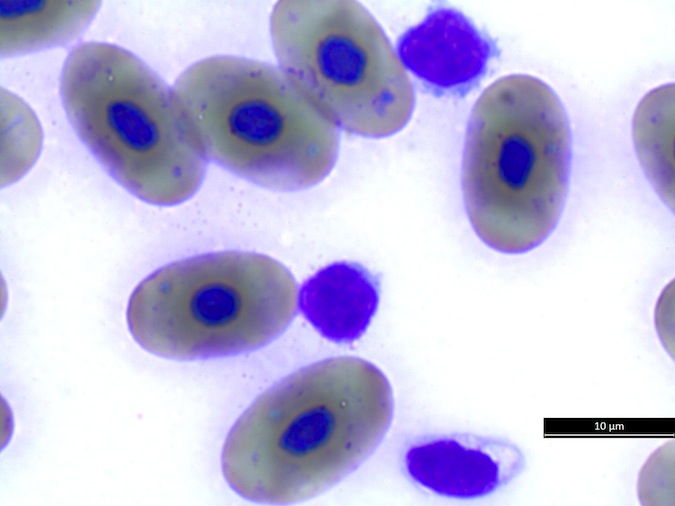
Basophil morphology is very variable in sea turtles (Fig. 8).
Figure 8. Basophil morphology in sea turtles. Wright-Giemsa stain.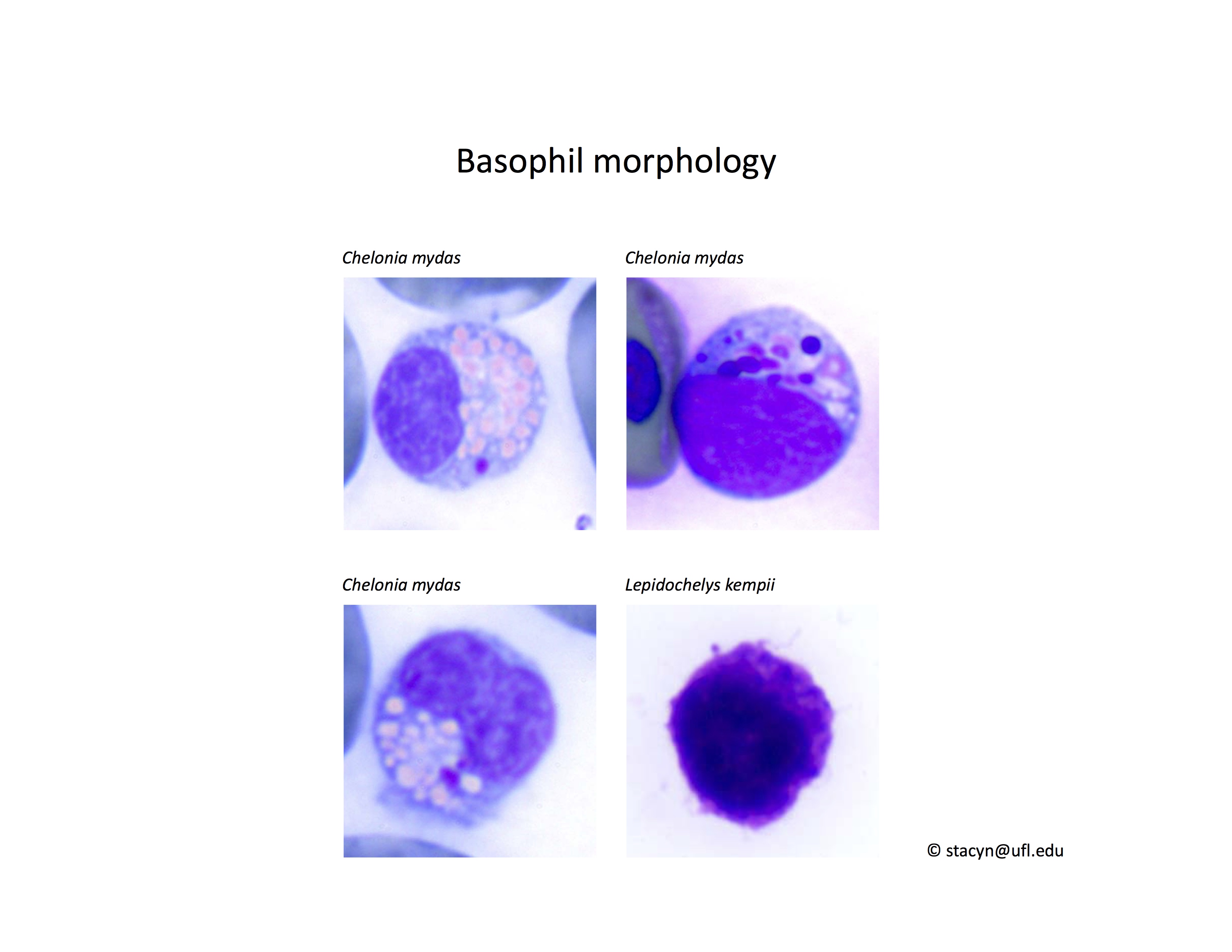
Monocytes (Fig. 9): Very low numbers of melanomacrophages or monocytes with proteinaceous debris are consistent with normal findings. However, monocytes with phagocytized erythrocytes and/or hemosiderin (greenish-basophilic, homogenous material) indicate red blood cell destruction. Phagocytosis of leukocytes by monocytes is also an abnormal, non-specific finding, which may indicate immune-mediated destruction of these cells in inflammatory disease. In cases of phagocytosis of cells in the peripheral blood, blood culture may be helpful to exclude septicemia. Please contact your microbiology laboratory to request incubation of reptile cultures at room temperature and for longer than for mammalian cultures.
Figure 9. Monocytes/Macrophages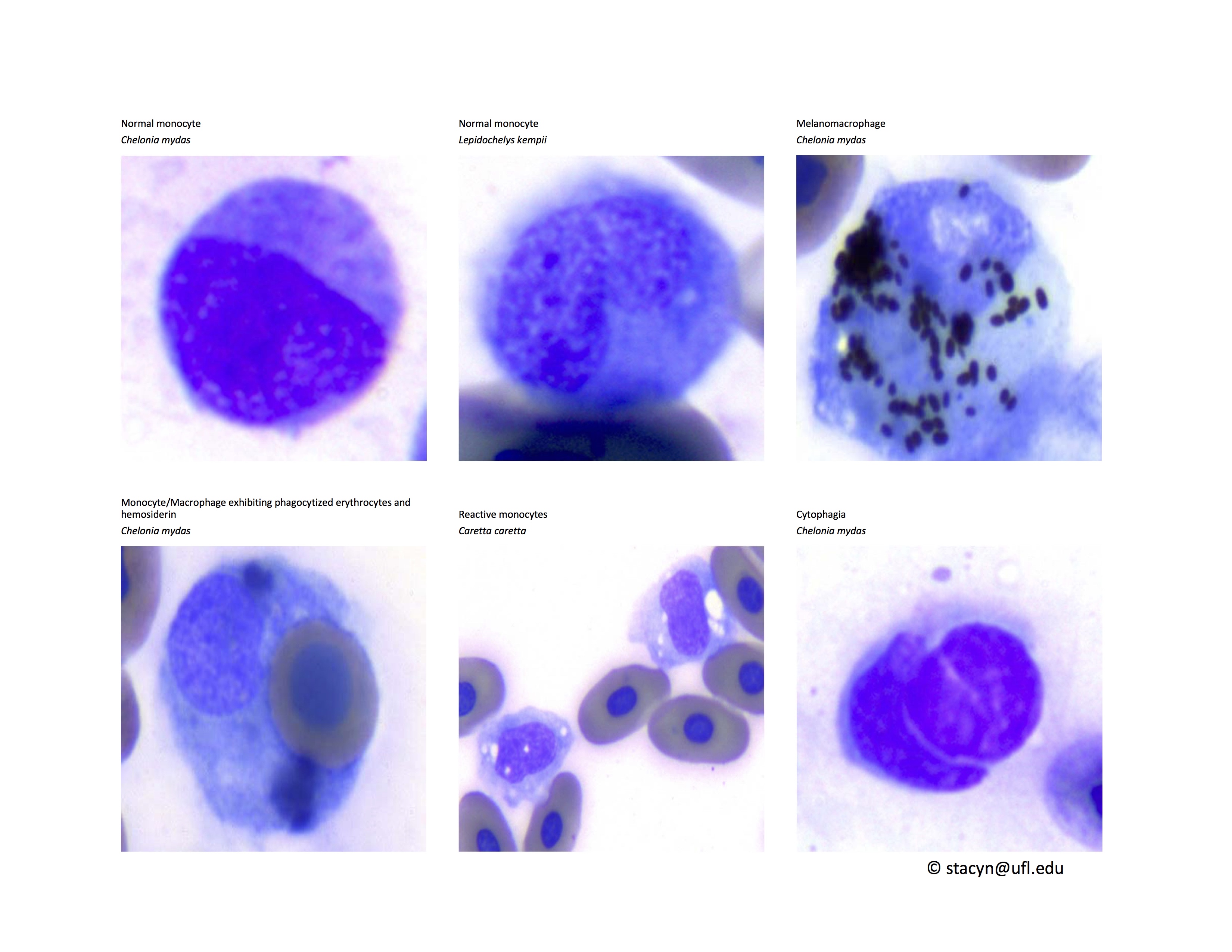
Lymphocytes (Fig. 10): As in other reptile species, lymphocytes need to be differentiated from thrombocytes. Lymphocytes can be reactive in response to antigenic stimulation. Reactive lymphocytes can have dark basophilic cytoplasm, and it can be difficult to differentiate them from early erythroid precursors. This differentiation is very important because the presence of a regenerative response in an anemic patient is a good prognostic indicator.
Figure 10. Lymphocytes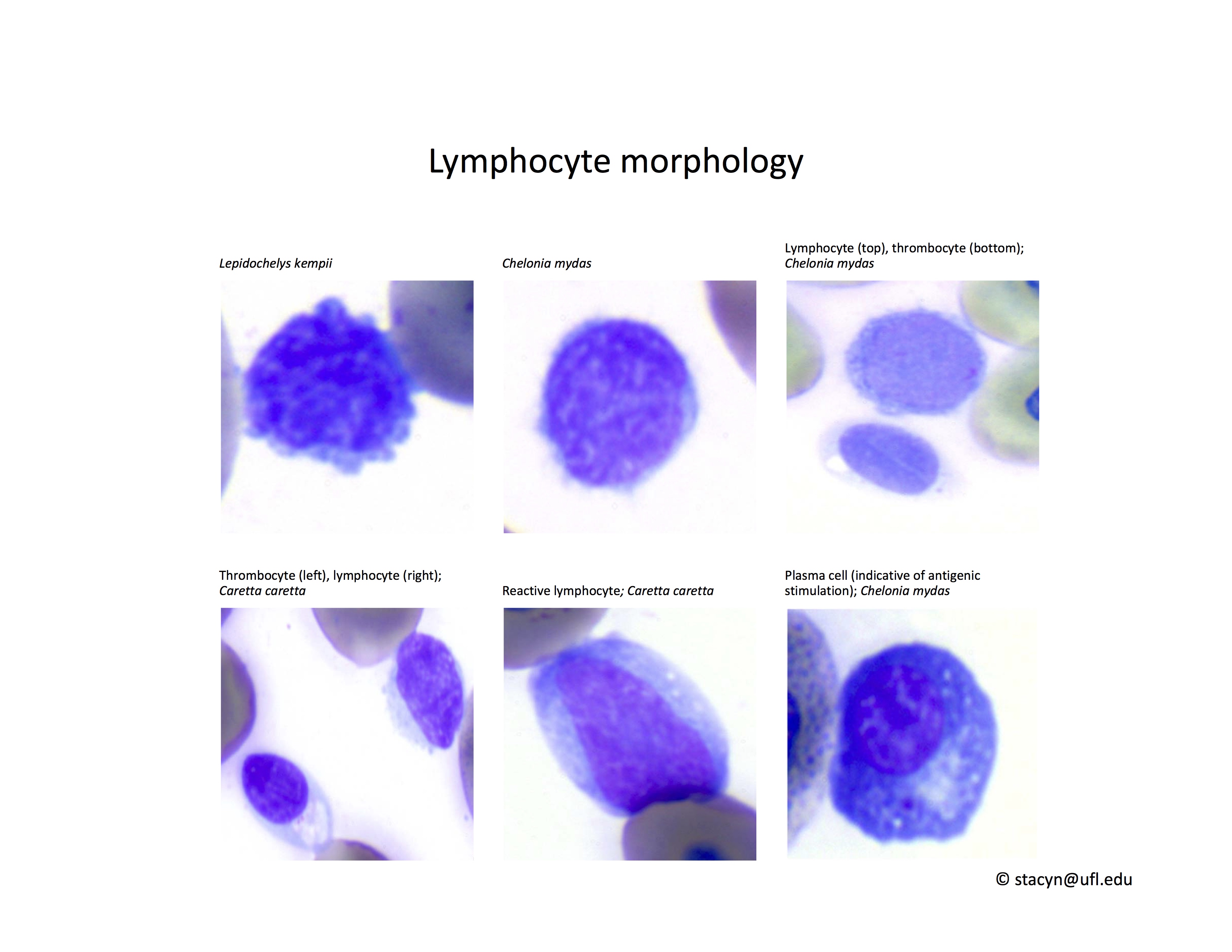
PLASMA BIOCHEMISTRY
Reference intervals are available for different sea turtle species with some considerations to life stage, gender, reproductive stage, or geography. Lymphatic fluid contamination can significantly affect hematology and biochemistry results (e.g., decreased PCV, WBC count, total protein, potassium, chloride). Lymph dilution should be considered upon aspiration of clear fluid during blood sample collection or if results are inconsistent with clinical findings.
The plasma color in sea turtles is typically straw-colored in healthy animals. Abnormal colorations of plasma include hemolysis, lipemia, or biliverdinemia (dark green plasma). Hemolysis can occur during difficult blood sample collection or inappropriate storage of the blood tube (e.g., direct contact of blood tube with wet ice) and significantly interferes with a number of chemistry analytes that need to be considered when interpreting chemistry results (esp. K, P, CK). Lipemia can be seen post-prandially and can be avoided by collecting fasted samples (8-12 hours). Light green color in green sea turtles or yellow plasma color may originate from dietary chlorophyll or carotenoids, respectively. Biliverdinemia (dark green color) has been associated with starvation or liver disease in sea turtles.
Nesting female sea turtles reportedly have higher concentrations of total protein, albumin, globulin, calcium, phosphorus, triglycerides, and cholesterol, associated with vitellogenesis and folliculogenesis as in females of other reptile species (Casal et al., 2009).
Analytes with diagnostic value in sea turtles:
Total Protein (TP), albumin (ALB), globulins (GLOB)
-
- Note: total solids obtained by refractometer may differ from total protein concentration, since glucose, urea, and sodium can increase the refractive index
- The most accurate method of protein measurement in reptiles is the biuret method with combined protein electrophoresis for determination of albumin and globulin concentrations (Osborne et al., 2010; Gicking et al., 2004; Macrelli et al., 2013)
- Hyperproteinemia: dehydration, inflammation; nesting females
- Hypoproteinemia/hypoalbuminemia can be associated with hypothermia, malabsorption/maldigestion, conditions of anorexia/inappetence in turtles affected by debilitation or severe disorders, decreased production by the liver, or loss from vascular space (incl. blood loss, protein loss through kidneys, gastrointestinal tract, or skin). Total protein and albumin are often used as criteria to determine whether a rehabilitated turtle can be released or not.
Uric Acid (UA)
-
- Mild increases may be noted with dehydration or post-prandially. Mild uric acid elevations can be associated with high dietary protein in green turtles fed animal protein during rehabilitation.
- Sea turtles excrete variable proportions of uric acid, ammonia, and urea
- Although uric acid is neither sensitive and nor specific in reptiles, this analyte appears closely regulated in sea turtles and elevated concentrations have been associated with renal disease (Stacy, unpublished data). Additional analytes may be abnormal in cases with renal disease (e.g., Mg, K, P).
Glucose (GLC)
-
- Hyperglycemia may be associated with stress, pain, iatrogenic administration (e.g., dextrose-containing fluids or dexmedetomidine), or exertion. It can vary with nutritional status and temperature in sea turtles.
- Hypoglycemia: hypothermia, malabsorption/maldigestion, debilitation, starvation, septicemia; nesting females due to exertion
ELECTROLYTES: sodium, chloride, potassium
-
- Sea turtles have salt glands that regulate peripheral electrolyte concentrations
- Sodium and chloride can be very useful in the assessment of the hydration status in sea turtles in conjunction with total protein, PCV, and a physical exam. Hypoalbuminemia can create peripheral edema (including eye turgor) despite elevated electrolytes from dehydration. The combination of dehydration and edema can be clinically difficult to recognize, and biochemical analytes (including electrolytes, total protein, albumin) can be supportive in the assessment of affected patients. Indications for appropriate fluid therapy in such patients need to be very carefully considered.
Sodium (Na) and Chloride (Cl)
-
- Hypernatremia and hyperchloremia: can be observed with dehydration or salt gland disorders (i.e. salt gland adenitis; Oros et al., 2011)
- Hyponatremia: overhydration (iatrogenic fluid administration, freshwater bath), loss through gastrointestinal tract, kidneys, and salt gland pathology
Magnesium (Mg)
-
- Hypermagnesemia is caused by decreased kidney excretion
- Interpretation in context with clinical findings and biochemistry (uric acid, TP, Na, Cl, P) to assess hydration status and to evaluate renal function
Potassium (K)
-
- Falsely increased potassium concentrations can be caused by in vitro hemolysis, difficult blood withdrawal, or prolonged contact of erythrocytes with plasma
- Potassium alterations often indicate changes in diet, disorders in acid-base balance, hypothermia, renal or gastrointestinal disease
Calcium (Ca) and Phosphorus (P): the concept of Ca:P ratio needs to be very carefully applied to sea turtles as it is heavily dependent on diet, season, and size of the turtle
-
- Ca and P imbalances can be seen with temperature, diet, or husbandry. Elevations in both Ca and P can occur with bone pathology (trauma or disease).
- Hypercalcemia: may be observed with granulomatous disease and nesting females with folliculogenesis
Creatine phosphokinase (CK)
-
- Elevations indicate muscle damage
- May be seen with exertion in stranded sea turtles, muscle damage from intramuscular injections, restraint, trauma, infectious causes, frostbite, or difficult venipuncture
Aspartate aminotransferase (AST)
-
- Wide tissue distribution
- Concurrent elevations of CK and AST are indicative of muscle damage which is common in stranded turtles (esp. cold-stunning)
Cholesterol (CHOL) and Triglycerides (TRIG)
-
- Variations may be associated with diet, or elevated concentrations related to folliculogenesis in nesting female sea turtles (Casal et al., 2009)
Blood urea nitrogen (BUN)
-
- Has been reported to increase during rehabilitation with high protein diets
- Decreased BUN is associated with herbivory in C. mydas or decreased protein intake in omnivorous/carnivorous sea turtles (Innis et al. 2009)
- Maybe used as an indicator for current feeding status
Iron (Fe)
-
- The measurement of plasma iron concentration reflects the amount of iron bound to the iron-transporting protein transferrin, and thus the measurement of the peripheral iron concentration alone is unreliable in the assessment of total body iron stores. Other iron assays (e.g., ferritin) are species-specific and currently not available for reptiles. Thus the plasma iron concentration is the only available analyte in assessing the iron status in sea turtles. It is important to keep this limitation in mind when interpreting results.
- Hypoferremia can be caused by disorders that cause loss or decreased production of plasma proteins, iron deficiency (chronic external blood loss, decreased intake or intestinal absorption) or shift of iron to storage sites in inflammatory conditions as in anemia of inflammatory disease
- Hyperferremia can be associated with hemolysis (in vitro or pathologic), iatrogenic overload, or possibly hepatocyte damage
Analytes with low to no diagnostic value:
-
- Wide tissue distribution (Anderson et al., 2013); thus possibly seen with rapid growth of immature turtles while on captive diet (esp. AST)
Creatinine (CREA)
-
- Very low peripheral concentrations in reptiles, not useful
Bile Acids have neither been established nor validated in sea turtles
URINALYSIS in sea turtles is of limited diagnostic value since sea turtles do not concentrate urine, and urine often gets contaminated with fecal material when passing through the cloaca. However, in due consideration of the aforementioned, cytologic evaluation of the urinary sediment or cloacal contents may provide diagnostically useful information. For example, urinary casts, protozoa, metazoan eggs, leukocytes, or erythrocytes found in urine/feces may provide useful clinical information when combined with other clinical findings.
References and Resources
Link to Dropbox folder with references included in this introduction HERE
The Cornell University, College of Veterinary Medicine, online text book covering clinical pathology among various species: e-ClinPath
For information on how to obtain a blood sample see Sample Collection Techniques
Published reference intervals for sea turtle chemistries are available for different sea turtle species and populations (e.g., google scholar), results from a wild caught population are available from the University of Florida and Archie Carr Center for Sea Turtle Research - Hematocrit and Plasma Biochemical Data for Sea Turtles in Florida
This chapter should be cited: Stacy Nicole I., Boylan S. 2014. Clinical Pathology of Sea Turtles. In: Mettee, Nancy. 2014. Clinical Pathology. Marine Turtle Trauma Response Procedures: A Veterinary Guide. WIDECAST Technical Report No. 17. Accessed online [date].
Updated 3/2016