- Preface and Intent
- Admissions
- Analgesia and Anesthesia
- Clinical Pathology of Sea Turtles
- Death Criteria
- Debilitated Turtle Syndrome
- Euthanasia
- Formulary
- Hook Removal
- Husbandry
- Pain
- Parasites
- Physical Exam
- Radiographic Anatomy
- Radiographic Technique
- Release Criteria
- Sample Collection Techniques
- Selected Surgical Techniques
- Veterinary Supplies
- Wound Management / V.A.C. Therapy
- Future Topics
- Want To Know More?
Marine Turtle Trauma Response Procedures: A Veterinary Guide
Terry Norton and Nancy Mettee (Editors). 2020. WIDECAST Technical Report No. 20.
- Preface and Intent
- Admissions
- Analgesia and Anesthesia
- Clinical Pathology of Sea Turtles
- Death Criteria
- Debilitated Turtle Syndrome
- Euthanasia
- Formulary
- Hook Removal
- Husbandry
- Pain
- Parasites
- Physical Exam
- Radiographic Anatomy
- Radiographic Technique
- Release Criteria
- Sample Collection Techniques
- Selected Surgical Techniques
- Veterinary Supplies
- Wound Management / V.A.C. Therapy
- Future Topics
- Want To Know More?
Analgesia and Anesthesia
NOTE: This summary is intended to be a brief, basic orientation in sea turtle analgesia and anesthesia and is not complete. Please refer to references provided at the end of this section for further reading.
Analgesia is defined as the inability to feel pain. Anesthesia is defined as a general or local insensibility to pain; this loss of feeling permits performance of surgery and other painful procedures.
Sea turtles do indeed feel pain which can manifest as withdrawal, biting, slapping, and evasive responses that can be very dangerous to both the patient and handlers attempting to restrain the animal. More subtle signs of pain may include depressed appetite and lethargy, which can be observed in a variety of other conditions that might be non-painful. In addition, pain has many physiologic responses which can affect anesthesia, surgery, and healing. For example, catecholamine release in response to pain may increase blood pressure, heart rate, and induce cardiac arrhythmias. Additionally, increased cortisol may increase blood glucose and may suppress the immune system. For these reasons, chemical restraint with analgesics is preferred for any procedure that is likely to cause pain.
Reptiles are ectothermic; thus, all their bodily functions are dependent on environmental temperature. The blood circulation in a reptile is extremely variable depending on the activity level prior to anesthesia, overall health status, and especially the environmental and core body temperature of the turtle. Additionally, sea turtles have physiologic dive responses (shunting of blood away from limbs and the ability to hold their breath for a significant amount of time) that will affect the uptake and removal of anesthetics. For these reasons, whenever possible the intravenous route is preferred over SQ or IM and mask induction is not feasible. Thermal support during anesthesia and recovery will result in improved drug metabolism and provide more rapid recoveries. A good target temperature for pre-, intra- and post op should be approximately 80° F (26° C). Most importantly large fluctuations in temperature during this period should be avoided. Water circulating heating pads, hot dog heating pads, and Bair huggers set at the appropriate temperature are all options that work well. Regular monitoring of the body temperature of the turtle pre-, intra-, and post-op is important. Caution should be exercised to avoid placing electrical heating elements beneath the patient as fluid leakage may cause an electrical short and subsequent burn. Electric blankets should never be used as they will overheat a reptile.
Sea turtles are pachyostotic and osteosclerotic meaning that their bone density increases with size and age. This will affect drug dosing as anesthetics typically do not distribute into bone. For this reason, smaller turtles will typically require higher end drug doses than larger turtles.
Eye lubrication is standard as with other species undergoing anesthesia to prevent the cornea from drying out.
Intubation
Intubation of sea turtles is best accomplished after induction with injectable anesthetics when there is sufficient loss of jaw tone. Tube size should be selected to minimize leakage but not be too tight of fit to avoid damage to the tracheal mucosa. Only uncuffed tubes should be used in sea turtles because they have complete tracheal rings and mucosal damage will occur with an inflated cuff. The end of the tube should be lightly lubricated and should slide smoothly into the glottis and never be forced. The tube can be introduced between the arytenoid cartilages located at the base of the tongue. The tip can be used to gently open the epiglottis. The tube is best introduced when the turtle takes a breath. The trachea bifurcates at the base of the heart and splits into two primary bronchi that run the length of the lung. The split comes early so caution should be used when inserting the tube to make sure it does not go too far. The head and neck should be a straight as possible so the end of the tube does not push on the tracheal mucosa which may lead to trauma and subsequent tracheal occlusion or obstruction.
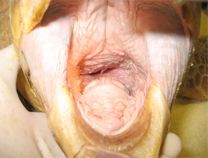
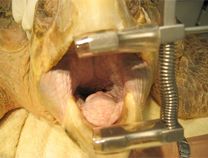
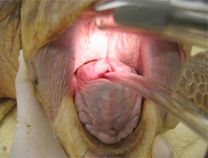
Waterproof tape should be used to secure the tube in place. A mouth gag should be left in place for the entire procedure to ensure the tube is not bitten by the patient. A soft mouth gag such as a Nyla bone or toothbrush handle wrapped in bandage material may be used as the initial mouth gag. Once the tube is in place, an appropriately sized PVC pipe may be taped in place as shown in the video.
Premeds
-
Meloxicam: Greens 0.5 mg/kg SQ SID x 5 days, Kemps 1 mg/kg q 12 hrs x 5 days, Loggerheads not effective, other sea turtle species have not been evaluated (information is based on preliminary results from pharmacokinetic (PK) study conducted by one of the authors TMN, Norton et al, in press)
-
Ketoprofen is the preferred NSAID for loggerheads 2 mg/kg q 24 hrs x 5 treatments (based on a published PK study, Thompson et al, 2018)
-
Tramadol 5 mg/kg q 48 hrs orally or SQ or 10 mg/kg q 72 hrs orally or SQ, can use long term without side effects (based on PK study conducted and published by one of the authors, Norton et al, 2015)
-
Hydromorphone 0.5 to 1 mg/kg SQ/IM q 12-24 hrs, may cause respiratory depression, no PK studies in sea turtles but based on red eared slider efficacy study (Kinney et al, 2011).
-
Premeds can be given the day before surgery so they are on board during surgery and then should be given post operatively. An NSAID and tramadol or hydromorphone can be used in combination for multimodal analgesia.
-
Very low doses of Dexmedetomidine IV (5 to 25 micrograms/kg) may be analgesic and can be used for debridement and other minor procedures and be reversed with equal volumes of atipamezole IV. Drug can be given IM or intranasally, but effects will take longer.
Local Analgesia
-
Lidocaine 6 mg/kg total dose (>10 mg/kg can be toxic) for local blocks around FP tumors, skin biopsies, incision sites, etc. Calculate total dose and do not use more.
-
Bupivicaine 1 mg/kg for local blocks, keep dose under 2 mg/kg.
Intrathecal Analgesia
-
Lidocaine (must be preservative free) 2-4 mg/kg IT in caudal vertebrae. Provides 30 to 60 min of analgesia in pre-femoral area, tail, cloaca, etc. (Mans et al. 2011).
Induction
-
Propofol 3-7 mg/kg IV (given slowly), can top up with 3 mg/kg IV if patient starts to wake up or intubate (no analgesic effects) (MacLean et al. 2008).
-
or
-
Midazolam 0.2 to 1 mg/kg IV (can give IM but not preferred) + Dexmedetomidine 50-100 micrograms/kg IV (IM/IN)
-
or
-
Ketamine 1-2 mg/kg IM/IV + Dexmedetomidine 50-100 mcg/kg IM/IV +/- Butorphanol 0.4 mg/kg IM/IV
-
or
-
Alfaxalone 5 mg/kg IV or IM (no analgesic effects) (Phillips et al. 2017).
Maintenance
Sevoflurane has minimal patient benefit over isoflurane and cost remains high for this inhalant anesthetic. Sevoflurane is preferred when possible over isoflurane because incidental exposure of humans to sevoflurane carries a reduced risk over isoflurane. Isoflurane, however, remains a good option for sea turtle anesthesia.
The drive for respiration is hypoxia and hypercapnia. As part of the dive response, sea turtles are extremely tolerant of both. Because of this, turtles will have no spontaneous respirations during anesthesia requiring intermittent positive pressure ventilation for maintenance of anesthesia. This may be done by a ventilator or manually. Breaths should be every 10 seconds with pressures not exceeding 20 mmHg while the turtle is becoming anesthetized. The frequency of respirations and the percent of gas anesthesia can be decreased over the course of the surgical procedure. A sigh every 5 minutes is recommended (where the lungs are inflated, and the pressure held for 5 seconds before release). Evaluation of the tidal volume can also be done by visualizing the patient’s body movement, but this will be challenging if the patient is in dorsal recumbency.
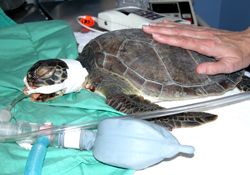
Use palpebral response, cloacal tone, and tail avoidance to evaluate depth of anesthesia. Typically, it is appropriate to begin surgery when a tail pinch fails to elicit a response and the palpebral response has been lost.
Length of surgery will vary but begin to decrease the concentration of the gas anesthetic gradually after the halfway point has been reached. It is typical to have turtles breathing strictly oxygen during closure of the incision. After the gas anesthesia is complete, the patient should be left on oxygen for 15-60 minutes during which IPPV is continued but the frequency of respirations is slowly decreased to one per minute. After this, the turtle should remain intubated on room air. An AMBU-bag may be used to continue IPPV, but the frequency may be further decreased to one breath every 1 to 5 minutes. Maintaining constant temperature throughout the procedure (80° F or 27° C) is ideal. Massage of the limbs to improve circulation. The turtle should be closely supervised while intubated. The authors have found measuring venous pH and PCO2 pre- and post op can be very helpful on decision making for continuation of ventilation and frequency of ventilation. If a patient is slow to recover, the pH is often low and PCO2 is high. Sequential pH and PCO2 levels can help determine patient progress. Ideally pH should be approximately 7.4 to 7.6.
The sea turtle cardiovascular and respiratory systems influence anesthetic choice, effectiveness and recovery. Sea turtles have a three chambered heart with two separate atria and one anatomically continuous ventricle. The ventricle is divided into two main chambers by a muscular ridge, which originates from the ventral ventricular wall and runs from the ventricular apex to the ventricular base. The two main chambers; the cavum pulmonale and the cavum dorsale, are similar in function to the right and left ventricles of mammals, respectively. The dorsolateral border of the muscular ridge is open, permitting the flow of blood between the cavum pulmonale and cavum dorsale. During ventricular systole the muscular ridge presses against the dorsal wall of the ventricle and separates the cavum pulmonale from the cavum dorsale. This allows the heart to act as a two-circuit pump. Reptiles, in general, have the ability of intracardiac shunting, which preferentially directs most of the blood flow to either the systemic or the pulmonary circulation. Intracardiac shunting stabilizes the oxygen content of the blood during respiratory pauses and directs blood flow away from the lungs during breath holding. Further, the right-to-left shunt is partly responsible for raising body temperature by increasing systemic blood flow to the periphery for uptake of heat from the environment. Cardiac shunting is common when sea turtles dive. Cardiac shunting can affect the uptake and elimination of inhaled anesthetics, as well as the systemic arterial oxygen content. The size and direction of the shunts are likely controlled by pressure differences between the pulmonary and systemic circuits. The pressure differences are principally controlled by cholinergic and adrenergic factors that regulate the vascular resistance of the pulmonary and systemic circulation. Large right-to-left shunts (bypassing the lungs) limit the amount of inhalant anesthetic uptake early in the anesthetic period and slow anesthetic elimination at the end of anesthesia. Therefore, these shunts may delay the induction and recovery of patients undergoing inhalant anesthesia. Conversely, a left-to-right shunt (bypassing the systemic circulation) will limit the distribution of injectable drugs to its site of action, as well as decrease the absorption and metabolism of the agent. Changes in the amount and direction of shunts may be responsible for the unexpected awakening observed in some sea turtles anesthetized with inhalant anesthetics. Epinephrine at 0.1 mg/kg IM or IV may decrease anesthetic recovery time when inhalants are used because it may lessen right-to-left shunts (Balko et al. 2018).
Sea turtle “CPR” can be used to help flush residual anesthetic from deep in the lung fields. See page 33 in Marine Turtle Trauma Response Procedures: A Field Guide for a description of the procedure.
Monitoring
Intravenous access facilitates administration of anesthetic agents and to manage this complication more effectively. Intracardiac shunts also have implications for patient monitoring; in particular, airway gas monitoring, capnography, and blood gases may be helpful in assessing the changes that occur with shunting. It will affect EKG interpretation as a single peak pulse wave will be seen and not a typical PQRS display. Peripheral pulses are not palpable and cardiac auscultation is difficult due to the presence of the external shell.
Cardiac evaluation is possible with a Doppler flow meter, EKG or ultrasound. A Doppler flow meter is used with the transducer placed over a major vessel.

Doppler flow meter
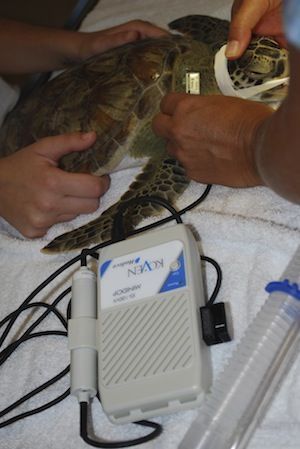
Dorsal Cervical Vessel

Ventral Cervical Vessel
EKG lead II placement will yield a pulse wave only, not a PQRS wave. Lead placement close to the body and with metal attachments to patient by skin staples or hypodermic needles can enhance the EKG trace in smaller patients.
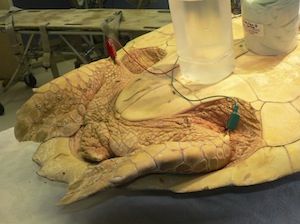
Ultrasound can visualize movement of the heart, left and right aortic arches, and pulmonary arteries using the left and right cervicobrachial area as a window.
Reflexes
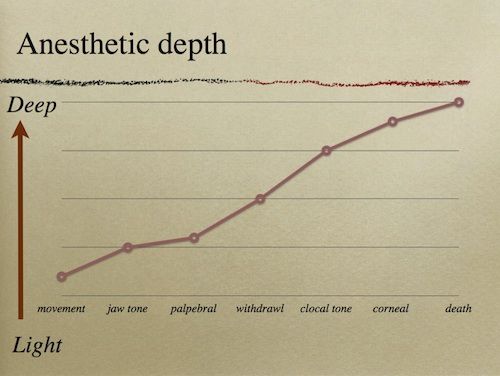
Recovery frequently involves several spurts of activity followed by rest. Do not extubate until at least three strong recovery spurts have been noted. If possible, delay extubation until the turtle is alert and having regular spontaneous respirations.
Following anesthesia, all patients are dry docked until they demonstrate sufficient strength. The amount of time needed prior to placing back in the water will depend on the procedure and the individual turtle’s response. A small tub should be used to prevent the turtle from excessive activity while out of the water. Once ready to be tested start in very shallow water and make sure the turtle can clear for a breath. Constant observation is needed until the turtle is back to normal behavior.
For further information suggest the following reading material:
Baker, B.B., K.K. Sladky, and S.M. Johnson. 2011. Evaluation of the analgesic effects of oral and subcutaneous tramadol administration in red-eared slider turtles, J. Am. Vet. Med. Assoc. 238: 220-227.
Balko, J.A., B. Gatson, E.B. Cohen et al. 2018. Inhalant anesthetic recovery following intramuscular epinephrine in the loggerhead sea turtle (Caretta caretta), J. Zoo Wildl. Med 49: 680-688.
Chittick, E.J., M.A. Stamper, J.F. Beasley, G.A. Lewbart, and W.A. Horne. 2002. Medetomidine, ketamine, and sevoflurane for anesthesia of injured loggerhead sea turtles: 13 cases (1996–2000), J. Am. Vet. Med. Assoc.221: 1019-1025.
Goe, A.M., J. Shmalberg, B. Gatson, P. Bartolini, J. Curtiss, et al. 2015. Effects of parenteral epinephrine and GV-26 stimulation on inhalant anesthesia recovery time in two orders of reptiles. In: Proceedings of the 22nd annual conference of the Association of Reptilian and Amphibian Veterinarians, San Antonio, Texas, 29-2 August-September, p.527-528.
Gornik, K.R., C.G. Pirie, R.M. Marrion, J.N. Wocial, and C.J. Innis. 2015. Baseline corneal sensitivity and duration of action of proparacaine in rehabilitated juvenile Kemp's ridley sea turtles (Lepidochelys kempii), J. Herpetol. Med. Surg. 25: 116-121.
Harms, C.A., S.A. Eckert, S.A. Kubis, M. Campbell, D.H. Levenson, et al. 2007. Field anesthesia of leatherback sea turtles (Dermochelys coriacea), Vet. Rec. 161: 15-21.
Harms, C.A., W.E.D. Piniak, S.A. Eckert, and E.M. Stringer. 2014. Sedation and anesthesia of hatchling leatherback sea turtles (Dermochelys coriacea) for auditory evoked potential measurement in air and in water, J. Zoo Wildl. Med. 45: 86-92.
Kinney, M., S.M. Johnson, and K.K. Sladky. 2011. Behavioral evaluation of red-eared slider turtles (Trachemys scripta) administered either morphine or butorphanol following unilateral gonadectomy, J. Herp. Med. Surg. 21: 54-62.
MacLean, R.A., C.A. Harms, and J. Braun-McNeill. 2008. Propofol anesthesia in loggerhead (Caretta caretta) sea turtles, J. Zoo Wildl. Med. 44: 143-150.
Mans, C., L.L. Lahner, B.B. Baker, S.M. Johnson, and K.K. Sladky. 2012. Antinociceptive efficacy of buprenorphine and hydromorphone in red-eared slider turtles (Trachemys scripta elegans), J. Zoo Wildl. Med. 43: 662-665.
Mans, C., P.V.M. Steagall, L.L. Lahner, S.M. Johnson, and K.K. Sladky. 2011. Efficacy of intrathecal lidocaine, bupivacaine, and morphine for spinal anesthesia and analgesia in red-eared slider turtles (Trachemys scripta elegans). In: Proceedings of the 43rd Annual Conference of the American Association of Zoo Veterinarians, Kansas City, Missouri, 22-28 October, p135.
Moon, P.F., and E.K. Stabenau. 1996. Anesthetic management of sea turtles, J. Am. Vet. Med. Assoc. 208: 720-726.
Mosley, C.A. 2005. Anesthesia and analgesia in reptiles, Semin. Avian Exot. Pet. Med. 14: 243-262.
Mosley, C.A. 2011. Pain and nociception in reptiles, Vet. Clin. North Am. Exot. Anim. Pract. 14: 45-60.
Mosley, C.A. and C.I. Mosley. 2015. Chapter 42: Comparative Anesthesia and Analgesia of Reptiles, Amphibians, and Fishes, in Veterinary Anesthesia and Analgesia, 5th ed., Grimm, K.A., L.A. Lamont, W.J. Tranquili, S.A. Greene, and S.A. Robertson (Eds.). Wiley and Sons Inc., Ames, Iowa.
Nardini, G., S. Silvetti, I Magnelli, N. Girolamo and M. Bielli. 2014. Medetomidine-Ketamine-Midazolam and Butorphanol (MKMB) as intramuscular injectable combination for anesthesia in loggerhead sea turtles (Caretta caretta), Veterinaria (Cremona) 28: 27-31.
Norton, T.M., C.I. Mosely, K.K. Sladky, et al. 2017. Chapter 3: Analgesia and Anesthesia, in Sea Turtle Health and Rehabilitation, Manire C., T.M. Norton, B.A. Stacy, C.J. Innis, C.A. Harms (Eds.). J. Ross Publishing. Plantation, Florida.
Norton, T.M., S. Cox, S.E. Nelson, M. Kaylor, R. Thomas, et al. 2015. Pharmacokinetics of tramadol and o-desmethyltramadol in loggerhead sea turtles (Caretta caretta), J. Zoo Wildl. Med. 46: 262-265.
Norton, T.M., and M.T. Walsh. 2012. Sea turtle rehabilitation, in Fowler's Zoo and Wild Animal Medicine Current Therapy, 7th ed., Miller, R.E., and M.E. Fowler (Eds.). Elsevier Saunders, St. Louis, chap. 31.
Phillips, B.E., L.P. Posner, G.A. Lewbart, E.F. Christiansen, and C.A. Harms. 2017. Assessment of the effects of three doses of intravenous alfaxalone on yearling loggerhead sea turtles (Caretta caretta). J. Am. Vet. Med. Assoc. 250(8): 909-917.
Sladky, K.K., V. Miletic, J. Paul-Murphy, M. Kinney, R. Dallwig, et al. 2007. Analgesic efficacy and respiratory effects of butorphanol and morphine in turtles, J. Am. Vet. Med. Assoc. 230: 1356-1362.
Sladky, K.K., and C. Mans. 2012. Clinical analgesia in reptiles, J. Exot. Pet Med. 21: 158-167.
Sladky, K.K. 2014. Chapter 18: Analgesia, in Current Therapy in Reptile Medicine and Surgery, 3rd ed., Mader, D.R. and S. Divers (Eds.). Elsevier-Saunders, St. Louis, MO.
Thompson KA, M.G. Papich, B. Higgins, et al. 2018. Ketoprofen pharmacokinetics of R-and S-isomers in juvenile loggerhead sea turtles (Caretta caretta) after single intravenous and single-and multidose intramuscular administration. J. Vet. Pharmacol. Ther. 41(2): 340-348.
Vigani, A. 2014. Chapter 22: Chelonia Tortoises, Turtles, and Terrapins, in Zoo Animal and Wildlife Immobilization and Anesthesia, Second ed., West, G., D. Heard, and N. Caulkett (Eds.). Blackwell Publishing Professional, Ames, Iowa.
Wyneken, J., D.R. Mader, E.S. Weber and C. Merigo. 2006. Chapter 76: Medical care of sea turtles, in Reptile Medicine Surgery, 2nd ed., Mader, D.R. (Editor). Elsevier Press, St. Louis, MO.